Tcelna™
TcelnaTM is an autologous T-cell immunotherapy being developed for the treatment of Multiple Sclerosis (MS), and is specifically tailored to each patient's immune response profile to myelin. Tcelna is designed to reduce the number and/or functional activity of specific subsets of myelin-reactive T-cells (MRTC) known to attack myelin. Opexa believes the potential combination of efficacy, superior safety, improved tolerability and patient-friendly administration schedule may position Tcelna as the MS treatment of choice as compared to existing therapeutic options.
Fast Track Regulatory Approval
Tcelna has been granted Fast Track status by the U.S. FDA in Secondary Progressive MS (SP-MS) based on the unmet need of the progressive indication and the potential of Tcelna to benefit this patient population. Opexa has also completed formal End of Phase II meetings with the FDA and has received support to move forward with pivotal Phase III clinical trials in Relapsing Remitting MS (RR-MS). Opexa is currently conducting a Phase IIb clinical trial in SP-MS patients and continues to evaluate its plans in the RR-MS indication.
ImmPath™ Manufacturing Process
Tcelna is manufactured using Opexa Therapeutics' proprietary method for the production of an autologous T-cell product, ImmPathTM, which encompasses the collection of blood from the MS subject, isolation of peripheral blood mononuclear cells (PBMC), generation of an autologous pool of myelin-reactive T-cells (MRTC) raised against selected peptides, and the return of these expanded, irradiated T-cells back to the subject. These attenuated T-cells are reintroduced into the subject via subcutaneous injection to trigger a therapeutic immune response consisting of anti-idiotypic cytotoxic and regulatory T-cells, to limit the frequency and function of T-cell dependent autoimmunity directed against myelin.
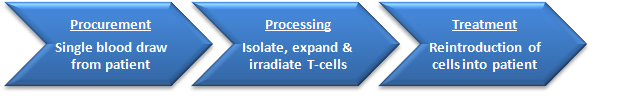
Tcelna is administered in an annual course defined as five subcutaneous injections over 6 months. Subjects will be treated with a new, personalized treatment course each year based on their altered disease profile or epitope shift and the re-manufacture of a new Tcelna product representing the emerging immunodominant T-cell response to myelin.
Tcelna Differentiation
Tcelna possesses a unique mechanism of action that combats the demyelination of the nerve fibers in the central nervous system, the underlying cause of MS. Five clinical trials have been completed with Tcelna in 356 patients, many with multiple years of treatment. Opexa believes Tcelna may possess a number of advantages compared to other MS therapies currently available or in development, including:
Personalization - Tcelna is a personalized autologous immunotherapy that is manufactured for every individual patient, tailored to his or her disease profile. Each patient receives a newly personalized treatment on an annual basis based on their unique epitope profile. Opexa is unique in that it evaluates 109 peptides across the three key myelin proteins to identify each patient's set of dominant antigens.
Efficacy - Clinical trials conducted to date demonstrate that Tcelna may result in a reduction in the Annualized Relapse Rate (ARR) for patients with MS, slowing of disease progression and evidence of an improvement in disability in a number of MS patients, which could suggest a neuroprotective benefit.
Safety and Tolerability - It is believed that Tcelna treatment selectively targets and regulates the pathogenic T-cell population. It is not a general immune suppressant and, accordingly, is not associated with the serious side effects seen by those MS treatments that function by systemically suppressing the immune system. In clinical trials conducted to date, the safety profile of Tcelna has remained superb.
Improved Compliance - Currently, available therapies are administered monthly and, in some cases, daily. The treatment regimen for Tcelna consists of only five subcutaneous injections per year, which we believe may provide compliance benefits to patients and physicians.
Clinical Development in SP-MS
Patients with Secondary Progressive MS (SP-MS) represent approximately 50% of the total MS population, yet treatment options remain very limited. Currently, there is only one product specifically approved for patients with SP-MS, and that product carries a black box warning due to severe cardiotoxicity. In an effort to meet this significant unmet medical need, Opexa is currently conducting a Phase IIb clinical trial of Tcelna in SP-MS patients.